Influence of the surface of contact on the velocity of chemical reaction
The reactions we considered before were homogeneous, i.e. they proceeded in
a homogeneous environment, for example, in a mixture of gases or in a solution.
But there are processes which take place on the contact surface between the reagents
(heterogeneous reactions), i.e:
- Solid substance and gas: S + O2 = SO2,
- Solid substance and a liquid: Fe + 2HCl = FeCl2 + H2,
- Two immiscible liquids: C3H7Br + KCN
(water) = C3H7CN + KBr(water)
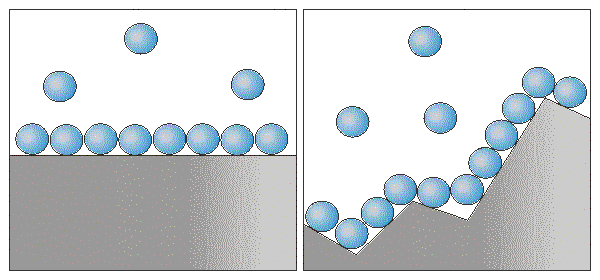
The velocity of heterogeneous reaction is directly proportional to the contact surface of reagents.
But there are some nuances.
*Solid substances that participate in heterogeneous reaction are reduced to small
fragments to increase the surface area of particles, thus increasing the velocity of interaction.
For example, coal for preparation of gunpowder is pounded in a powder. A liquid is pulverized for reaction with
gas : as diesel fuel (a mix of hydrocarbons) is injected in the chamber where it combusts with the air.
Supplementary
| 



