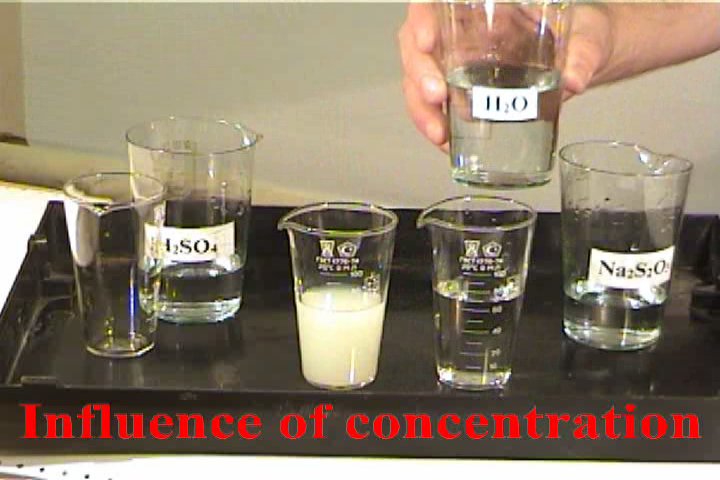
Influence of concentration of reagents
on the velocity of chemical reaction.
The experiment is based on the reaction:
H2S2O3
= S + SO2 + H2O

Formation of white-yellowish cloud (insoluble
sulfur) is observed. Sulfacid is unstable (look
at the reaction), that's why it has to be obtained
by interaction between sodium thiosulfate and
diluted solution of sulfuric acid.
Na2S2O3
+ H2SO4 = H2S2O3
+ Na2SO4
Summary reaction:
Na2S2O3
+ H2SO4 = S + SO2
+ H2O + Na2SO4
How to carry out reaction: Place 20
ml of 2M sulfuric acid in 2 identical glasses.
Add 80 ml of water in the first glass to dilute
the acid. Simultaneously pour in both glasses
(from other 2 glasses or cylinders) 20 ml 2M of
sodium thiosulfate.
What to observe: In which glass the
cloud of sulfur is formed faster?
Influence of a contacting surface of
reagents on the velocity of chemical reaction.
The experiment is based on the reaction:
Mg + 2H2O = Mg(OH)2
+ H2
What is observed - water solution of phenolphtalein
becomes pink as a result of its interaction with
magnesium hydroxide.
How to carry out reaction: Pour 5 ml
of water into 2 identical test-tubes and add 1
drop of phenolphthalein in each. Add magnesium
filings to the first test-tube, magnesium powder
- to the second. If it is necessary (if the powder
is old) heat the test-tubes.
What to observe: In which test tube
pink color appears earlier and its intensity increases
faster?
Influence of the character of reagents
on the velocity of chemical reaction.
The experiment is based on the reaction
of alkaline metals with water:
M + H2O = MOH + 1/2H2
What is observed - water solution of phenolphtalein
becomes pink as a result of alkaline formation,
and liberation of hydrogen bubbles.
How to carry out reaction: Pour some
water in large vessel and add some drops of phenolphthalein
solution. Clean a little piece of lithium with
filter paper and carefully place it in the water
using a pincer. When lithium reacts fully repeat
this experiment with sodium, and then with potassium.
What to observe: In which reaction intensity
of liberation of hydrogen is greater?
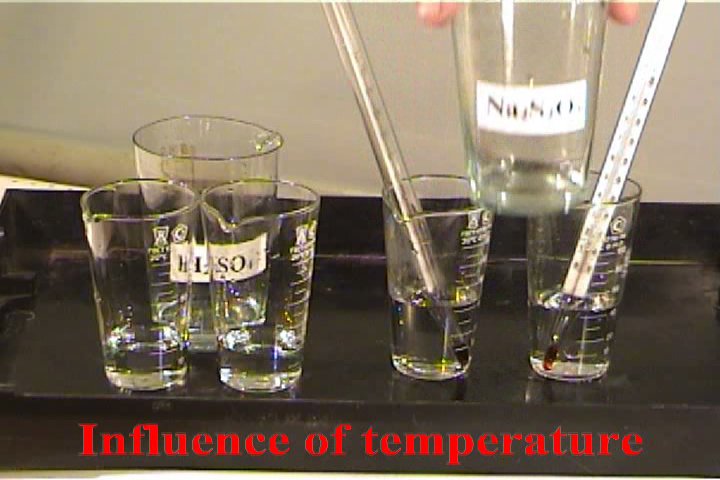
Influence of temperature on the velocity
of chemical reaction
The experiment is based on the reaction:
H2S2O3
= S + SO2 + H2O
Formation of white-yellowish cloud (insoluble
sulfur) is observed. Sulfacid is unstable (see
the reaction), that's why it has to be obtained
by interaction between sodium thiosulfate and
diluted solution of sulfuric acid.
Na2S2O3
+ H2SO4 = H2S2O3
+ Na2SO4
Summary reaction:
Na2S2O3
+ H2SO4 = S + SO2
+ H2O + Na2SO4
How to carry out reaction: Pour 20 ml
of 2M sulfuric acid in 2 identical glasses . Heat
one of the glasses on the hot plate. Simultaneously
pour in both glasses (from 2 other glasses or
cylinders) 20 ml 2M of sodium thiosulfate.
What to observe: In which glass the
cloud of sulfur is formed faster?
Catalysis
The experiment is based on the reaction
of the decomposition of hydrogen peroxide
H2O2 =
H2O + 1/2O2
that is accelerated at the presence of MnO2,
salts of heavy metals and some enzymes. What is
observed - liberation of gas bubbles, in which
wooden stick burns brightly.
How to carry out reaction: Pour 10 ml
of 30% H2O2 in high cylinder
(100 ml) . Quickly add some of MnO2
powder. Bring in the cylinder smouldering wooden
stick.
-
Catalysis
The experiment is based on the reaction
of catalytic oxidation of ammonia on chrome oxide.
4NH3 + 5O2
= 4NO + 6H2O
What is observed - sparks (red-hot particles
of chrome oxide are formed due to the exothermal
effect of reaction).
How to carry out reaction: Rinse carefully
within big flat-bottomed flask (500 ml) with concentrated
solution of ammonia (thus inside we get high concentration
of ammonia vapors). Throw in it heated powder
of chrome oxide (III) using an iron spoon.






