Influence of temperature on the velocity. Additional material.
The rule of Vant-Hoff
The rule of Vant-Hoff is no more than rule it has no validity of the law.
First, Vant-Hoff could study chemical reactions in the bounded conditions
which were provided by laboratory technical equipment of that time. As it was
found out later, the temperature factor can change in a significant temperature interval.
Besides because of imperfection of technical means it was impossible to study as
very fast reactions (proceeding in milliseconds), and very slow ones(for which thousand
years are required). Reactions with participation of greater molecules of the complex
form (for example, proteins) also do not submit to the rule of Vant-Hoff.
The energy of activation
Molecule is energetically advantageous formation (otherwise no molecules would exist).
It means, that chemical substances on the energy diagram occupy positions in "wells".
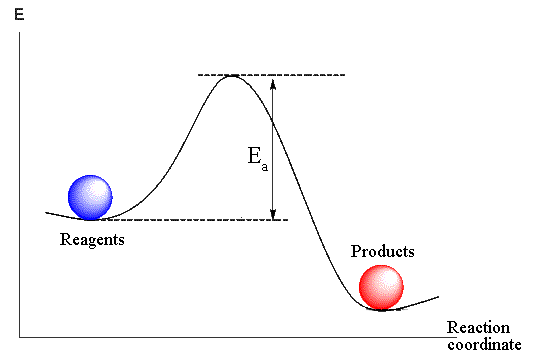
If we wish to carry out a reaction, i.e. to transform these substances into others, we should
give sufficient energy to the reagents to surpass the "barrier"
(energy of activation).
For this purpose, as a rule, it is required to heat up a reaction mix to the certain
temperature to increase the kinetic energy of molecules. If the energy barrier of a reaction
is low then an impact or friction is enough for its beginning.
Supplementary
| 



