Influence of the character of reagents on the velocity. Additional material.
As "the nature of reacting substances" we mean:
For substances of a molecular structure - types and durability of chemical bonds in molecules
of reagents. It is necessary for some bonds to be destroyed (partially) before the molecule can react. For substances of non molecular structure (an ionic or nuclear crystal)
- the structure of a crystal lattice, its durability. For substances, at which "molecule" consists of one atom (for example, metals, noble gases)
- the structure of an electronic environment of atom, durability of coupling with external electrons. For molecules of the complex form - probability of favourable positioning of reagents
for reaction at impact. In the left figure the impact will lead to reaction but in
the right one it will not, despite the strength of the impact.
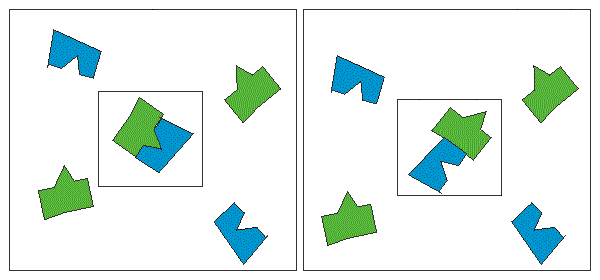
Last factor refers to as steric, or geometrical. It is especially significant for
reactions with participation of such greater molecules, as, for example, proteins.
The most of their reactions proceeds only at presence of enzymes. One of functions of
enzymes is in giving to reagents certain configuration that is convenient for interaction.
Supplementary
|




